Elizabeth Ndichu, MD, Duke Global Health Institute, Duke University School of Medicine, and Kevin Schulman, MD, Clinical Excellence Research Center, Department of Medicine, Stanford University
Contact: Elizabeth Ndichu, elizabethndichu@gmail.com
Abstract
What is the message?
Sampling retail pharmacy outlets, we were able to identify the high prevalence of poor-quality drugs (drugs that are mislabeled in terms of the amount of active medication or drugs that have high levels of impurities). We discuss potential market and regulatory solutions to addressing our findings.
What is the evidence?
We collected primary evidence on the prevalence of poor-quality medications across regions of one market. We examined factors related to the prevalence of poor-quality drugs including socioeconomic status of the neighborhood and the retail price of the product. Overall, we were struck that none of these factors were related to the prevalence of poor-quality medicines. We then explored how a range of interventions across the supply chain could help address the findings from our study.
Submitted: November 8, 2018; accepted after review: December 20, 2018.
Cite as: Elizabeth Ndichu, Kevin Schulman. 2019. Identifying and Solving the Problem Of Poor Quality Drugs. Health Management Policy and Innovation, Volume 4, Issue 2.
Introduction
Since the invention of modern medicine, the development and sale of pharmaceutical products has become widespread – and with the diffusion comes the problem of poor-quality drugs. Although many measures have sought to curb quality problems, trade in poor-quality drugs is still rife globally. Indeed, a pandemic of poor-quality drugs threatens both international trade and the health of populations. (1)(2)(3)
What constitutes poor-quality drugs? The World Health Organization (WHO) and others use a wide range of terms, including substandard genuine products that fail to meet pharmaceutical specifications, falsely labelled products that do not contain the ingredients claimed in their packaging, falsified products that misrepresent identity or source, and counterfeit drugs with or without appropriate active pharmaceutical ingredients (APIs) that are presented in inauthentic packaging. (4) (5) In this discussion, we will use two terms: falsely labelled and substandard. Falsely labelled drugs have excessively high or low amounts of APIs, while substandard drugs have high levels of impurities. In turn, we refer to drugs that are either falsely labelled or substandard as poor-quality drugs.
The scope of the problem of poor-quality drugs transcends national borders because the manufacturing and supply chain of medical products thrives in an international market.(6) Drugs manufactured in China, India, North America, and Europe, for instance, are often distributed and consumed throughout the world.
Are false labeling and/or impurities common?
In a bid to get a better understanding of the magnitude of the problem of poor-quality drugs, we carried out an analytic study in Lagos State, the second most populous state in Nigeria(7). Our aim was to identify the prevalence of poor-quality drugs and to investigate any association with socioeconomic status or drug pricing. With the rising number of premature deaths due to non-communicable chronic diseases, we sampled nifedipine, a drug used in the management of chronic hypertension, including management of angina, high blood pressure, Raynaud’s phenomenon, and premature labor. The drug is sold with the brand name Adalat, among others.
Using High Performance Liquid Chromatography (HPLC) in a lab at Campbell University in the U.S., we assessed the quality of drugs sold by drug stories in six Local Government Areas (LGAs) of Lagos State, Nigeria. Three of the LGAs have high socioeconomic status and three have low socioeconomic status. Within the 102 samples we collected, there were substantial issues with both false labelling and substandard quality.
False labelling: Unfortunately, false labelling was common, including 29.4% (30) of the samples. Based on the international pharmacopeia standards, the nifedipine API fell below the U.S. Food and Drug Administration (FDA) and USP lower limit of 90% in 29 cases and above the limit of 110% in one case. Of the 30 falsely labelled drugs, 56.7% (17) came from low socioeconomic status areas and 43.3% (13) from high economic status areas, showing that the issue occurs across economic classes.
Impurities: Impurities were even more common. Nifedipine nitrophenylpyridine analog impurities, which constitutes one of the two major impurities found in nifedipine tablets, exceeded the 2.0% specification in 74.5% (76) of the samples.(8) Of the drugs with high levels of impurities, unlike the falsely labelled cases, a higher proportion 40 (52.6%) came from high socioeconomic status areas and 36 (47.4%) were from low socioeconomic status LGAs. Again, then the issue spans economic status.
Pricing: We did not observe a difference in pricing between good and poor-quality nifedipine drug samples. Thus, it is not that the market could not support the cost of high-quality drugs. Instead, it is a market failure that consumers cannot demand high-quality products when they fill their prescriptions.
How might this affect health?
Impurities and false labelling can cause unwanted pharmacological and toxicological effects. Impurities can produce end-organ damage such as renal and liver failure. False labeling can lead to precipitated disease progression which in turn results in negative physiologic effects and treatment failure.(9) Non-communicable diseases such as hypertension are major killers in low- and middle-income countries (LMICs) – without good-quality drugs the management of these patients will be more complex and as a result, the number of deaths due to NCD’s will continue to increase. Moreover, the negative impact on health of poor-quality medicines imposes financial costs on consumers, which further reduces health seeking behavior and causes more premature deaths.(10)(11)
Fraud or poor manufacturing control?
A key question is whether the poor quality results from intentional fraud or from poor control of manufacturing. If the drugs were produced by fraudulent manufacturers, we would expect to find extremely high levels of impurities, with very low or complete lack of appropriate APIs. In our study, though, all samples had the right API, suggesting that the drugs came from legitimate manufacturers.
Clearly, we cannot rule out the possibility that fraudulent manufacturers produced some of the samples collected. Beyond visual inspection of the packaging, we were not able to measure the packaging, weigh the tablets and packets, scan packaging, or benchmark the products against original manufacturer products. Nonetheless, the most likely source of most of the problems appears to be manufacturing issues rather than intentional fraud.
What can be done and who needs to do it?
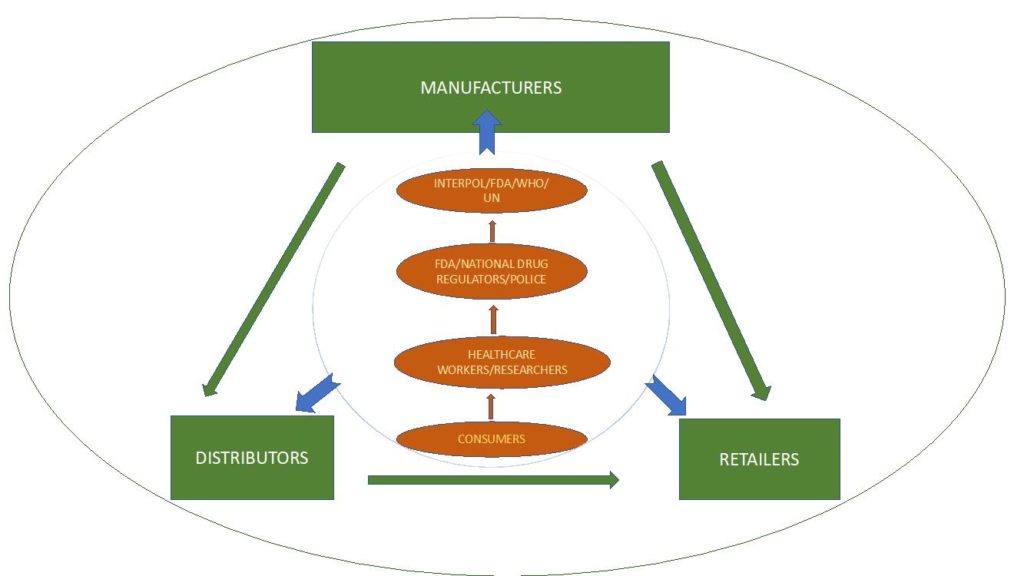
Trade in poor-quality drugs affects both traders and patients. Traders face reputational and financial risks from selling poor-quality products. Most critically, patients might follow management plans laid out by their providers only to suffer from the use of poor-quality drugs. We propose solutions to the problem of poor-quality drugs aimed at the full pharmaceutical value chain: consumers and clinicians; supply chain stakeholders including manufacturers, distributors, and retailers; and regulators. Figure 1 below illustrates the complex regulatory environment of the pharmaceutical supply chain.
Consumers and clinicians
Our findings provide evidence that consumers and healthcare providers need to create a demand for better quality products. Although clinicians are often at the forefront of identifying poor-quality drugs in the course of their practice, empowering patients to take charge of the investigation of the pharmaceutical supply chain is a cornerstone of tackling this problem from the demand side.
The first step in engaging patients and clinicians would be to raise awareness of this issue using mass media and the medical literature. Health education on the prevalence of poor-quality drugs will aid in engaging consumers in the fight against poor-quality drugs. From investigation of drug packaging to presentation of drug samples, encouraging consumers to provide suspicious samples to specified local public health officers, clinicians, researchers, and the police would increase the capacity of preexisting regulatory bodies and create a sense of ownership among users of pharmaceutical products. Leveraging mobile phone technology can create avenues for consumers to report suspicious products to regulatory bodies responsible for drug control, local police, researchers and health care workers. Such steps will result in visible demand for better products as retailers and other stakeholders in the pharmaceutical supply chain will be aware their goods are under close investigation.
If consumers and clinicians were informed on the extent of the problem, they could demand that pharmacies certify the quality of their products which could reverberate throughout the supply chain. Local newspapers could replicate studies like ours to help drive consumer engagement. Encouraging patients and clinicians to report adverse events emanating from consumption of substandard products will also aid in dramatizing the effects of poor-quality drugs.
Supply chain stakeholders
The pharmaceutical supply chain includes manufacturers, distributors, and retailers. Each of these critical market stages can be used to provide information about drug samples from production to consumption.
Manufacturers: Manufacturers should be held accountable through public and private regulatory schemes to ensuring that the products produced meet international quality control standards. In some cases, countries can inspect facilities with their own staff. Many LMICs, though, lack the ability to carry out inspection of international or even domestic facilities. In such cases, countries can leverage inspections by the U.S. FDA, European Medicines Agency (EMA), the WHO, and others. When facilities fail such international inspections, countries that lack their own inspectors can refuse sales from those facilities until they pass the international standards.
Producers also have a proactive role. Manufacturers can work toward building common quality databases with information about their products that can be made available to other stakeholders of the pharmaceutical supply chain as well as the public. This will ensure that awareness is raised on the quality of drugs and help create a transparent trading environment. Specific to the final product, manufacturers can employ basic technical solutions such as holograms, barcodes, and scratch-off numbers that can be used to uniquely identify authentic products.(1) Such fieldable solutions on packaging have been shown to play a key role in strengthening the security of the pharmaceutical supply chain, particularly in low resource settings. Such transparency will enable manufacturers of high-quality medicines to differentiate their products (differentiation would allow manufacturers to obtain both sales and price advantages in the market).
Distributors: Similar to manufacturers, distributors carry a significant responsibility in ensuring the quality of drug products. They could conduct their own testing on products to ensure their quality before they enter the local supply chain. Again, they could do this in a way that is transparent to consumers to help drive markets away from poor quality drug products.
In turn, distributors could maintain a database of production records, which can help sustain a transparent drug distribution system. Collaborating and sharing data adds depth to the understanding of the problem of poor-quality drugs since most traders target a wide range of products from varying companies. Distributors should also work to honor domestic and international licensing requirements. Such steps will work to the benefit of honest and effective distributors, helping drive out ineffectual and dishonest firms.
Retailers: Lastly, retailers are critical in ensuring the security of the pharmaceutical supply chain. Stocking only certified products is one major way in which they could drive up the quality of medications in the market. Pharmacists could also help inform consumers of their efforts to improve the quality of products they provide — and drive up their own sales by offering higher quality products to consumers.
Like their predecessors in the supply chain, retailers should work to ensure transparency on the quality of the medicines they stock. They should also work with local authorities, researchers, and the public at large to allow easy assessment of the pharmaceutical products being traded. Retailers should also vet the distributors and manufacturers they work with as a way of creating demand for good quality medicines.
Regulatory bodies
It is crucial to ensure manufacturing processes are well regulated and monitored as a means of addressing poor quality in manufacturing (our major finding) and also as a means of addressing fraudulent products (which is a considerable concern but not the subject of our study).
At the international level, regulatory bodies should collaborate and have common guidelines to help oversee the trade of drug products. As we discussed, we found that some manufacturers can produce high-quality products at the prevailing market price. Thus, one focus from a regulatory perspective would be to encourage all manufacturers to meet the same high-quality of manufacturing. Our evidence of the feasibility of achieving this quality standard in the retail market in Lagos should be a strong incentive for regulatory enforcement.
Most poor-quality products in our study were imported into the local market. Organizations such as INTERPOL, the WHO, the U.S. FDA, United Nations Office on Drugs and Crime, and the World Customs Organization should work cohesively in combating the trade of poor quality drugs. One major distinction is between poor manufacturing quality (a civil concern) and counterfeit products (a criminal concern). The regulatory bodies with jurisdiction for these two types of poor-quality products may or may not overlap, which is a challenge. Together, however, these organizations have a mandate to raise awareness across various countries about falsely labelled and substandard products. Organizations such as the FDA Office of Criminal Investigation follow up on violations of the Food, Drug, and Cosmetic Act in the United States. Creating similar organizations globally will provide and address the scope of poor-quality drugs in the world.
At a national level, the licensing of manufacturers, distributors, and retailers should be mandatory. Regulators at the national level should provide technical support to enable drug testing in the different stages of the supply chain. Governments and private sector stakeholders should work with researchers and consumers in carrying out drug quality investigations to ensure high quality. Stakeholders in the drug supply chain in each country should collaborate with international organizations such as INTERPOL and the U.S. FDA to investigate and confiscate poor quality or counterfeit drugs.
Additionally, although not the subject of our study, tax policy may also have an impact on the availability of poor-quality products. Taxes raise the price of medicines, enticing consumers to seek drugs from unregulated markets where they are lower priced. By helping reduce prices for legitimate goods through tax reductions, governments will reduce incentives on consumers to utilize these unregulated markets.
Conclusion
Our study found a substantial proportion of falsely labelled and substandard purity drugs. However, we found good-quality drugs were available for the same price as poor-quality products. This suggests that the high-prevalence of poor-quality products is not a result of inherent limitations in manufacturing medicines at an accessible price, but more likely is due to failures of oversight throughout the pharmaceutical supply chain. The results pose clear challenges for stakeholders throughout the healthcare system, from production to consumption, to identify and drive poor quality products from the market. This study also highlights the need for collaborative efforts in the screening and analysis of pharmaceutical drug products across all levels of the pharmaceutical value chain.
Author contributions: Dr Ndichu drafted the manuscript. All authors contributed to the study design, data collection and analysis, revision of the manuscript and final approval of the manuscript.
Declaration of interests: None.
Role of the funding source: Not applicable.
References
- Antignac M, Diop BI, Macquart de Terline D, Bernard M, Do B, Ikama SM, et al. Fighting fake medicines: First quality evaluation of cardiac drugs in Africa. Int J Cardiol. 2017 Sep 15;243(Supplement C):523–8.
- Koczwara A, Dressman J. Poor-Quality and Counterfeit Drugs: A Systematic Assessment of Prevalence and Risks Based on Data Published From 2007 to 2016. J Pharm Sci. 2017 Oct 1;106(10):2921–9.
- International Workshop on Counterfeit Drugs (1997: Geneva S, Policies WHOD of DM and, Drugs WAP on E. Report of the international workshop on counterfeit drugs, Geneva, 26-28 November 1997. 1998 [cited 2018 Jan 12]; Available from: http://www.who.int/iris/handle/10665/64157
- http://www.who.int/medicines/services/counterfeit/faqs/SSFFC_FAQ_print.pdf.
- WHO | Substandard and Falsified (SF) Medical Products [Internet]. WHO. [cited 2018 Jan 12]. Available from: http://www.who.int/medicines/regulation/ssffc/en/
- Cockburn R, Newton PN, Agyarko EK, Akunyili D, White NJ. The Global Threat of Counterfeit Drugs: Why Industry and Governments Must Communicate the Dangers. PLOS Med. 2005 Mar 14;2(4):e100.
- http://worldpopulationreview.com/world-cities/lagos-population/.
- Research C for DE and. Counterfeit Medicine [Internet]. [cited 2018 Jan 12]. Available from: https://www.fda.gov/Drugs/ResourcesForYou/Consumers/BuyingUsingMedicineSafely/CounterfeitMedicine/
- https://www.ncbi.nlm.nih.gov/books/NBK202526/.
- Ogah OS, Okpechi I, Chukwuonye II, Akinyemi JO, Onwubere BJ, Falase AO, et al. Blood pressure, prevalence of hypertension and hypertension related complications in Nigerian Africans: A review. World J Cardiol. 2012 Dec 26;4(12):327–40.
- https://www.who.int/gho/ncd/mortality_morbidity/en/.
- Zou W-B, Yin L-H, Jin S-H. Advances in rapid drug detection technology. J Pharm Biomed Anal. 2018 Jan 5;147:81–8.
References
- Antignac M, Diop BI, Macquart de Terline D, Bernard M, Do B, Ikama SM, et al. Fighting fake medicines: First quality evaluation of cardiac drugs in Africa. Int J Cardiol. 2017 Sep 15;243(Supplement C):523–8.
- Koczwara A, Dressman J. Poor-Quality and Counterfeit Drugs: A Systematic Assessment of Prevalence and Risks Based on Data Published From 2007 to 2016. J Pharm Sci. 2017 Oct 1;106(10):2921–9.
- International Workshop on Counterfeit Drugs (1997: Geneva S, Policies WHOD of DM and, Drugs WAP on E. Report of the international workshop on counterfeit drugs, Geneva, 26-28 November 1997. 1998 [cited 2018 Jan 12]; Available from: http://www.who.int/iris/handle/10665/64157
- http://www.who.int/medicines/services/counterfeit/faqs/SSFFC_FAQ_print.pdf.
- WHO | Substandard and Falsified (SF) Medical Products [Internet]. WHO. [cited 2018 Jan 12]. Available from: http://www.who.int/medicines/regulation/ssffc/en/
- Cockburn R, Newton PN, Agyarko EK, Akunyili D, White NJ. The Global Threat of Counterfeit Drugs: Why Industry and Governments Must Communicate the Dangers. PLOS Med. 2005 Mar 14;2(4):e100.
- http://worldpopulationreview.com/world-cities/lagos-population/.
- Research C for DE and. Counterfeit Medicine [Internet]. [cited 2018 Jan 12]. Available from: https://www.fda.gov/Drugs/ResourcesForYou/Consumers/BuyingUsingMedicineSafely/CounterfeitMedicine/
- https://www.ncbi.nlm.nih.gov/books/NBK202526/.
- Ogah OS, Okpechi I, Chukwuonye II, Akinyemi JO, Onwubere BJ, Falase AO, et al. Blood pressure, prevalence of hypertension and hypertension related complications in Nigerian Africans: A review. World J Cardiol. 2012 Dec 26;4(12):327–40.
- https://www.who.int/gho/ncd/mortality_morbidity/en/.
- Zou W-B, Yin L-H, Jin S-H. Advances in rapid drug detection technology. J Pharm Biomed Anal. 2018 Jan 5;147:81–8.
