Andrea Prado, Associate Professor, INCAE Business School; Benjamin Gallo Marin, Medical Student, The Warren Alpert Medical School of Brown University; Ramiro Casó, Senior Researcher, INCAE Business School
Contact: Andrea.prado@incae.edu
What is the message?
Speratum, a Costa Rican startup determined to find an effective therapy for pancreatic cancer, is operating effectively in a developing country, despite financial and non-financial challenges that life science enterprises face in this context.
What is the evidence?
Speratum, based in Costa Rica, is close to finishing its preclinical trials for a treatment for pancreatic cancer. Its work has been recognized internationally. Marín-Muller won the Dutch Innovation for Health and the Innovadores de América—Science and Technology category—prizes in 2018 and was the only Latin American invited to present his project in the Buckingham Palace among a group of 23 entrepreneurs from 15 countries around the world in the event Pitch at Palace.
Cite as: Andrea Prado, Ramiro Casó, Benjamin Gallo Marin. 2020. Speratum: Building a Life Science Startup in Costa Rica. Health Management, Policy and Innovation (www.hmpi.org), Volume 5, Issue 2.
Leading Edge Life Sciences Innovation In Costa Rica
Latin America is rarely perceived as a region where scientific discoveries are channeled through the complex pipeline required for their successful commercialization, particularly in the context of the life science startup. Nonetheless, while funding limitations complicate such endeavors, some Latin American life science startups have made dramatic progress towards the commercialization of their biomedical innovations.
This paper highlights the intriguing case of Speratum, a startup based in San Jose, Costa Rica, that is currently advancing its mission to provide a novel microRNA-based therapeutic for pancreatic cancer. We explore the challenges that Speratum faces in its geographic location and identify strategies that are forwarding the company’s commercialization goals. In doing so, we identify key lessons in the management of life science startups based in non-traditional contexts.
Pancreatic Cancer: The Need For Treatments
Speratum’s founder, Christian Marín Müller grew up in Costa Rica, where he developed an aptitude for science, particularly biology and chemistry. After witnessing family members and loved ones battling cancer, he was inspired to pursue a career in scientific research, with the hope to one day find a cure.
Christian, whose undergraduate training in biology directly exposed him to the challenges of commercializing novel biological products, decided to further his education with a graduate program in entrepreneurship and a focus on biotechnology. He went on to earn a PhD in Molecular Virology and Microbiology at Baylor College of Medicine (BCM) in Texas, where his graduate and postdoctoral training led to the development of new technologies that could potentially lead to better treatments for patients.
Pancreatic cancer
The pancreas is a tube-shaped organ located behind the stomach. It serves multiple key functions including the regulation of the metabolism and the control of blood glucose levels.1
Pancreatic cancer occurs when malignant cells grow unregulated to the point that tissues are structurally and functionally damaged. The disease accounts for about three percent of all cancers in the United States and about seven percent of cancer-related deaths. In 2020 in the United States, about 57,600 people will be diagnosed with pancreatic cancer and 47,050 people will die from it.2,3 Globally, pancreatic cancer is the 12th most common cancer in men and 11th most common in women.4
A 2017 study shows that pancreatic cancer cases worldwide have more than doubled since 1990.5 The average lifetime risk of suffering from pancreatic cancer is one in 64 and risk factors such as genetics, smoking, exposure to certain toxins, age, obesity, and diabetes, among others, can facilitate the onset of the disease.6,7
Often described as a “silent disease”, pancreatic cancer presents little to no symptoms during its earliest stages. As the cancer spreads, however, pain develops in the upper abdomen and symptoms such as weight loss, fatigue, and yellow discoloration of the eyes and skin may become apparent.8,9 Because pancreatic cancer is rarely detected during its earliest stages due to the absence of clinical symptoms, it is often diagnosed only after the disease has spread. The high mortality associated with pancreatic cancer is partially explained by the difficulty of obtaining an early diagnosis: less than one in 10 people are alive five years after being diagnosed with the disease.10
The discovery
In 2008, Christian initiated his PhD program at BCM in Houston, Texas. Christian worked under the supervision of Dr. Qizhi Cathy Yao, a prominent virologist and pancreatic cancer researcher. During his time in the Yao Laboratory, Christian investigated the role that a type of oligonucleotides called microRNAs (miRNAs) play in pancreatic cancer. miRNAs are small molecules that usually turn off the expression of specific genes.
In a healthy state, microRNAs play an important role in regulating a cell’s biological functions and gene expression. In a disease state, however, microRNAs can function abnormally and dramatically alter cellular behavior and gene expression.12 In some illnesses, restoring the equilibrium of microRNAs can help bring a cell from the disease state to a healthy state.
In 2013, Christian and colleagues published a paper in the journal Clinical Cancer Research that suggested that a molecule called miRNA-198 suppresses cancer-inducing genes in pancreatic cancer cells in the laboratory.11 Because miRNA-198 is not present in human pancreatic cancer cells, Christian hypothesized that finding ways to introduce miRNA-198 in these cells could reduce the expression of genes that facilitated the disease and theoretically, cure the cancer.
The finding that Christian made on miRNA-198 inspired him to investigate a treatment option for pancreatic cancer that used this molecule. Christian decided to put his discovery to the test of entrepreneurship. Christian knew that further studying this molecule held the potential to provide patients suffering from this disease with a new treatment method.
Speratum: A Life Science Startup
Getting started
In 2014, Christian formally founded the life science startup, Speratum, which means “hope” in Latin. He wanted to continue the preclinical phase of miRNA-198 research using animal models, while hoping one day to gain approval from the Food and Drug Administration (FDA) or European Medical Agency (EMA) and make the potential treatment option available worldwide. Christian set up Speratum in his native country, Costa Rica, a small Central American nation with a stable sociopolitical climate and a rich pool of professionals in the sciences.
In 2015, Christian began building the company. He first established a scientific board, joined by experts in pancreatic cancer, biotechnology investment, clinical research experts, and his two mentors at BCM, including Qizhi Yao. The next step was to build a strong scientific team. Christian hired Osvaldo Vega, a master’s student in biomedical sciences and genomics at the University of Costa Rica, who eventually became the company’s Chief Science Officer (CSO). Together, they recruited top talent for different roles, including chemists, biologists, veterinarians, medical doctors, and engineers.
Speratum also opened its doors to top students in Costa Rica from various universities to complete their academic theses with the company. Christian is proud that Speratum has been able to hire promising young scientists, thus offering an avenue for serious professional work for local scientists in the making. Most of them have stayed on at Speratum post-graduation, while others have gone on to train in nanotechnology and biotechnology at top institutions worldwide—with the goal of returning with that training, as Christian did with Speratum.
By January 2019, Speratum had grown into a team of 14 full time employees and several rotating students and collaborators. Overseen by a Board of Directors, a Board of Scientific Advisors, the CEO, CSO, and Project Manager, Speratum is organized into six departments: Nanoparticle Development, Microbiology, Molecular Biology, Toxicology, and Animal Facilities.
Melvin Nuñez, who has been serving as Project Manager since November 2018, comments that the entire team—including Christian—meets weekly to discuss the results of each scientific experiment performed and the administrative needs of the startup. Christian estimates that he spends 60 percent of his time working on science, 25 percent on networking and fundraising for the company, and 15 percent on high level administrative tasks.
A disciplined experimental mindset
Juan Carlos Valverde, Scientific Leader of Molecular Biology, states that the company encourages its scientists to “replicate each experiment multiple times”, in order to confirm the validity of results. Scientific rigor is juxtaposed with freedom of scientific creativity, in some cases born out of a desire to conduct cost-effective drug development.
Many of the company’s most creative endeavors have led to new intellectual property and novel applications for its technologies. For instance, the company is currently preparing a spin-off technology to treat cancer in dogs, born out of a desire to generate a low-cost production system for miR-198 that resulted in a manufacturing approach that can generate a low-cost alternative for the veterinary cancer market. Speratum’s resourcefulness plays an important role in the dramatic progress the company has seen in the preclinical research phase. Resources have been strategically allocated, and a culture of innovation has been implemented that has led to Speratum being considered an innovation leader in the region, with participation in some of the world’s largest technology entrepreneurship and cancer research forums.
Effective efficiency
Due to its location, Speratum can be highly cost effective. Although the company’s operating monthly expenses are “highly dependent on the experiments being performed”. These costs represent “about a fourth of what [they] would be spending if the entire operation would take place in the United States”.
Speratum has made dramatic progress in the preclinical phase with a fraction of the funding that most enterprises in drug development enjoy. Of 10,000 compounds with promising early results, only 250 successfully complete early preclinical testing, and only five enter clinical trials. Only one in those five drugs entering the clinic will make it to market, following a rigorous human testing regimen.
For example, Mirna Therapeutics based in Austin, Texas raised millions of dollars in venture capital starting in 2007 to fund oligonucleotide research for cancer. Their preclinical work alone had a cost of about $4 million per year. However, their product proved to be too toxic and the company ceased their investigations and the assets were acquired in 2017.
By contrast, Speratum has obtained encouraging data about their oligonucleotide product and has spent under $2 million in a span of roughly five years. The decision to focus on a highly promising molecule and practicing strategic resourcefulness has allowed Speratum to make great strides in their central project while keeping costs as low as possible.
Challenges of Building a Life Science Startup in Developing Countries
The recruitment
After Christian patented miRNA-198, the Costa Rican newspaper La Nación wrote about his discovery in September 2013. As a result, Christian states that “a lot of people invited [him] to Costa Rica to interview”. One of the individuals that contacted Christian was Allan Boruchowicz, founder and managing director of Carao Ventures, one of two venture capital firms that operate in Costa Rica and invests in early-stage Latin American startups. Christian states that after introducing miRNA-198 to Carao, “they asked [him] if [he] would like to start a business with them in Costa Rica, and the first thing that [he] told them is that it would be impossible in this country”.
Despite his early concerns about the location, Christian’s enthusiasm about Costa Rica grew after meeting with Franklin Chang-Diaz, a renowned Costa Rican scientist, entrepreneur, and former NASA astronaut. Chang-Diaz had established a company in Liberia, Costa Rica to develop novel propulsion technologies for deep space travel. Chang-Diaz told Christian that “[he] had the potential to change many things in the country, and that [he] should seriously think about conducting his scientific work in Costa Rica”. Christian became convinced to further pursue the development of this technology in Costa Rica and fuel scientific innovation in his home country.
Costa Rica: Benefits and challenges for life sciences startups
Costa Rica is a small Central American nation bordered by Nicaragua and Panama. With a population of about 4.9 million, the country is a unitary presidential constitutional democracy and enjoys a stable sociopolitical culture. Costa Rica is known for its highly educated population: in 2016, the government spent roughly 6.9 percent of its budget on education, versus a global average of 4.4 percent.13,14 In recent years, the Costa Rican economy has diversified to include sectors such as finance, ecotourism, and the production of medical devices. Since Costa Rica is part of the Free Trade Zone (FTZ), foreign manufacturing and services companies can benefit from investments and tax incentives.15
In addition to the benefits of a highly skilled workforce and lower labor costs, Costa Rican entrepreneurs face important limitations that make it difficult to thrive in the life sciences. Key constraints include the need to import reagents and raw materials for research, as well as the challenge of maintaining small business status despite not having any sales or products in the short to middle term horizon. A major challenge is that the government offers little research funding to companies. While grants do exist, they are limited, and often times the regulations are such that one company cannot compete for multiple grant opportunities.
Life science companies seeking to perform research must rely primarily on angel investors and venture capital funds. However, it is difficult to attract foreign investors: while Costa Rica is well known for developing medical devices, it is not regarded as a hub for bringing novel drugs and pharmaceuticals to the global market. In addition, Christian comments that the lack of an investment track record in biotechnology, with its high risk and long-term returns, makes investment from locals in companies like Speratum more difficult.
Moreover, high level investors in Europe and the U.S. are often reluctant to invest in a Costa Rican based entity, not having a frame of reference for success in health-based biotech endeavors in the region. Christian believes this is a hurdle that can be overcome with early successes in the industry, such as the filing of a successful Investigational New Drug (IND) from Speratum, to foment biotechnology investment growth for the region.
Initial funding
Carao Ventures provided Christian with the opportunity to raise $800,000 in initial funding, as well as provided office spaces, business support, and legal advice. Allan Boruchowicz commented in 2016 in La Nación that Speratum was interesting to Carao Ventures because “if the science is effective, not only will [they] obtain a great return but [they] also will have contributed in the development of a revolutionary drug that will save many lives, [which] has a big value for [them] and [their] investors”.16
Speratum and Carao Ventures offered their investors convertible notes, which is a strategy often used when it is difficult to define a company’s value in a given moment in time. Speratum’s future value—which can vary widely in the tens of millions of dollars depending on the results of their science—will depend on whether their research shows promising results for safety and efficacy leading up to and including to an IND approval. Essentially, at a later point in time, investors can choose to transform their investment into shares. If Speratum’s value is deemed to be high, the company will issue its shares at a premium. Christian argues that this agreement is “ultimately fair for those investing in this risk”.
Finding a laboratory
For some time, “the idea of the enterprise was to establish the company in Costa Rica but outsource a majority of the science abroad with the right people, assuming that [in Costa Rica] we would find limited infrastructure for conducting the necessary experiments”. Christian mentions that he “spent a lot of time — almost an entire year—evaluating all the possibilities for Speratum”. Christian and Carao Ventures came to the conclusion that the human capital in Costa Rica consisted of top-notch researchers who were more than capable of conducting the science and at a reduced cost in labor expenses. Therefore, the cheapest and most promising option would be to conduct experiments in the country. It would be critical, however, to find adequate laboratory space.
During the year-long process of due diligence on the capabilities within the country, Franklin Chang-Diaz connected Christian with Sergio Madrigal, who was the director of the Centro Nacional de Biotecnológicas (CENIBiot) in San Jose. The CENIBiot was funded with the support from the Costa Rica Government, CONARE (Consejo Nacional de Rectores) and the European Union with a donation of 11 million Euros to establish a world class laboratory for biotechnological innovations.
When Christian met Madrigal, “the CENIBiot was looking for a renaissance” and was interested in bringing new projects on board. Christian was able to negotiate the terms of his laboratory space contract, resulting in favorable conditions “with the caveat that [Speratum] will support Costa Rican innovation and it will make contributions to the country and [CENIBiot]” by bringing cutting edge science and collaborative programs to Costa Rica.
After signing a preliminary contract and beginning to operate at the CENIBiot, Speratum’s relationship with the Center has evolved to the extent that “[the organizations] apply to grants together and [are both] working on a nanotechnology project, as well as building together a unique animal facility”. Speratum has recently signed a new contract with CENIBiot that will “serve as a strategic alliance for both organizations, outlining how to develop independent projects that benefit both sides and a system to generate reciprocal licenses.” Christian describes Speratum’s relationship with CENIBiot as “a very interesting symbiosis”.
Speratum’s preclinical development requires working with specialized laboratory rodents as established models for cancer research and toxicology. Christian was interested in establishing a world-class animal facility at CENIBiot. Christian and the CENIBiot team had to spend considerable time obtaining legal permits from Costa Rican authorities and educating logistics and customs processing companies on animal importation protocols in order to establish a safe and effective route to import these specialized animals into Costa Rica.
As part of establishing a facility that could support Speratum’s research needs, Christian initiated the process to establish necessary paperwork, protocols, and infrastructure to receive accreditation from the Assessment and Accreditation of Laboratory Animal Care. The AAALAC is a private non-profit organization based in the United States that promotes the humane and ethical treatment of research animals. A government grant for $35,000 helped make obtaining the grant possible—although later prevented Speratum from being able to apply for additional grants. Besides making Speratum the first organization to receive such accreditation in Central America, AAALAC accreditation is significant to the company because “it represents quality, promotes scientific validity and assurance in a global marketplace, and demonstrates accountability”.17 Speratum received AAALAC accreditation for its animal care and use program in May 2019.
Christian Marín Müller: “If we succeed, we could change more than just lives”
When it comes to why Costa Rica, Christian is perfectly clear: even though multiple challenges exist, the possibility of succeeding could bring change not only to the lives of patients with pancreatic cancer at a global scale but also to a country and its entrepreneurial ecosystem. “We found incredible talent, we have the support of important actors in the local ecosystem, such as Franklin Chang and Carao Ventures; we also have the support of important people who have been part of the medical research industry for decades. Now we have this fantastic accreditation and created an animal facility that did not exist. In sum, we are doing things that have never been done in the region”.
Future Challenges and Lessons Learned
Next steps for Speratum
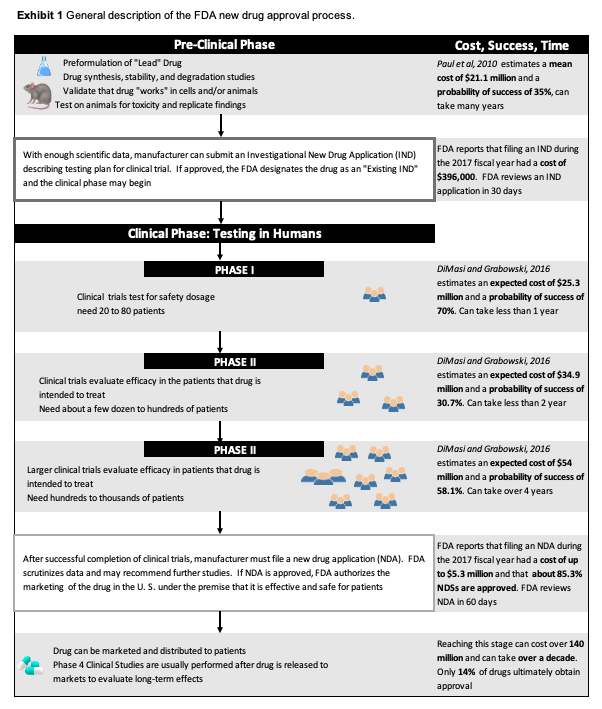
Christian and his team remain hopeful that their novel therapeutic will prove to be safe and efficacious and be launched to the global market, but, first, they must continue to perform the studies to meet the requirements for FDA and EMA approval. The ultimate goal remains years ahead as the company is close to finishing the preclinical investigation phase and making plans to successfully execute the very expensive clinical phases (Exhibit 1).
Although in February 2019 Speratum secured an additional $2 million in funding, Christian knows that Speratum will need far more capital to continue its progress. The difficulty of raising funds, particularly in attracting foreign investors to Costa Rica, and other limiting factors have made accelerating Speratum in Costa Rica challenging. Christian is proud Speratum has done well in Costa Rica with the preclinical research phase almost complete, despite a relatively limited budget. As a result, Speratum’s leadership has considered establishing a presence for Speratum abroad.
Where will the money come from?
As we have shown, operating a life science startup from a developing country has its pros and cons. Limited financial opportunities create an obvious challenge. Access to local research grants tend to be non-existent or available only for local academic institutions rather than for companies.
An alternative to public financial support, accessing resources through investors, faces challenges in both global and domestic funding. Globally, international investors are often concern about intellectual property issues when investing in startups registered in developing countries. Domestically, the number of sophisticated investors in developing countries who might be familiar with supporting life science startups is low. In Central America, for instance, most investors channel their resources either through real estate projects or traditional stock markets or financial instruments.
Life science startups have a relatively high probability of failure and a long term pay off—if any. Thus, startups like Speratum are more likely to raise financial resources in ecosystems where more investors have some degree of expertise in this industry. Thus, a life science startup from these countries has few options, especially after its preclinical research when resources needed for clinical trials become significant.
Institutional challenges and the need to find local resources
Adequate infrastructure and regulatory procedures are a challenge in developing countries that have not had experience with life science startups. Infrastructure is often concentrated in public universities and governmental institutions, which limit access for private companies. After a failed approach to obtain laboratory space at a public university, Speratum was fortunate to negotiate a deal for the rental space at CENIBiot, an institution that had just received resources to set up a world class laboratory for biotechnological innovations. Nevertheless, Christian still had to drive the process to receive the AAALAC accreditation for its laboratories.
Developing countries also need to develop specific regulations and procedures to operate new ventures. For example, importing inputs for laboratory research often requires the entrepreneur to coordinate efforts with governmental institutions to develop the necessary processes. Hence, life scientists and entrepreneurs operating in developing countries often need to engage more in administrative and networking efforts than if the enterprise were to operate in a developed country.
Operating in a developing country might be significantly less expensive than in a developed country, but ventures need to obtain high quality staff and other resources. In Costa Rica, access to highly skilled human resource was not a limitation for Speratum, but it will be in other developing countries. Life science enterprises require highly specialized professionals, equipment, and infrastructure, as well as a supportive ecosystem where governments, academia, and investors provide the resources they need to operate successfully. Nonetheless, entrepreneurs can sometimes find pathways to coordinating players and resources within a local ecosystem that others have not recognized.
Looking Forward
Christian is emotionally tied to Costa Rica. His aspiration to revolutionize the landscape of scientific innovation in his home country is serious. It has taken extraordinary efforts to make operations there possible, such as paving the way in working with local authorities about what life science enterprises need to succeed and then identifying solutions that address those needs. Christian wants to see the company’s future pancreatic cancer therapy succeed with as much of the development done in Costa Rica as possible. But as much as he would like for it to be entirely investigated in Costa Rica to be given to the entire world, he states that, “at the end of the day, the most important thing is that the treatment reaches people as quickly and effectively as possible”. Although, over time, this will require both a local and global presence, Costa Rica and its resources will remain a cornerstone of Speratum’s strategy.
Exhibit 1 General description of the FDA new drug approval process.
Source: See references 18-20
References
- Gittes, G. K. Developmental biology of the pancreas: a comprehensive review. Dev. Biol. 326, 4–35 (2009).
- Pancreatic cancer statistics. World Cancer Research Fund https://www.wcrf.org/dietandcancer/cancer-trends/pancreatic-cancer-statistics (2018).
- Siegel, R. L., Miller, K. D. & Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 70, 7–30 (2020).
- Aier, I., Semwal, R., Sharma, A. & Varadwaj, P. K. A systematic assessment of statistics, risk factors, and underlying features involved in pancreatic cancer. Cancer Epidemiology vol. 58 104–110 (2019).
- Pourshams, A. et al. The global, regional, and national burden of pancreatic cancer and its attributable risk factors in 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. The Lancet Gastroenterology & Hepatology 4, 934–947 (2019).
- Raimondi, S., Maisonneuve, P., Löhr, J.-M. & Lowenfels, A. B. Early onset pancreatic cancer: evidence of a major role for smoking and genetic factors. Cancer Epidemiol. Biomarkers Prev. 16, 1894–1897 (2007).
- Lowenfels, A. B. & Maisonneuve, P. Epidemiology and risk factors for pancreatic cancer. Best Pract. Res. Clin. Gastroenterol. 20, 197–209 (2006).
- Holly, E. A., Chaliha, I., Bracci, P. M. & Gautam, M. Signs and symptoms of pancreatic cancer: a population-based case-control study in the San Francisco Bay area. Clin. Gastroenterol. Hepatol. 2, 510–517 (2004).
- Porta, M. et al. Exocrine pancreatic cancer: symptoms at presentation and their relation to tumour site and stage. Clin. Transl. Oncol. 7, 189–197 (2005).
- Yadav, D. & Lowenfels, A. B. The epidemiology of pancreatitis and pancreatic cancer. Gastroenterology 144, 1252–1261 (2013).
- Marin-Muller, C. et al. A tumorigenic factor interactome connected through tumor suppressor microRNA-198 in human pancreatic cancer. Clin. Cancer Res. 19, 5901–5913 (2013).
- Li, M. et al. MicroRNAs: control and loss of control in human physiology and disease. World J. Surg. 33, 667–684 (2009).
- OECD. OECD Economic Surveys: Costa Rica 2018. (OECD Publishing, 2018).
- Gonzales, M. THE ROLE OF EDUCATIONAL LEADERSHIP ON PARTICIPATION IN THE NATIONAL PROGRAM OF SCIENCE AND TECHNOLOGY FAIRS OF COSTA RICA. EDULEARN17 Proceedings (2017) doi:10.21125/edulearn.2017.0291.
- Monge González, R., Rosales Tijerino, J. & Arce Alpízar, G. Cost benefit analysis of the free trade zone system: the impact of foreign direct investment in Costa Rica. http://www.sidalc.net/cgi-bin/wxis.exe/?IsisScript=earth.xis&method=post&formato=2&cantidad=1&expresion=mfn=019609 (2005).
- L, M. V. Tico buscará apoyo europeo para terapia contra cáncer de páncreas. La Nación, Grupo Nación https://www.nacion.com/tecnologia/innovaciones/tico-buscara-apoyo-europeo-para-terapia-contra-cancer-de-pancreas/FYDZCTSQ5FAVPBS6WXUYEOGJEU/story/ (2016).
- What is AAALAC Accreditation? AAALAC https://www.aaalac.org/accreditation-program/what-is-aaalac-accreditation/.
- Paul, S. M. et al. How to improve R&D productivity: the pharmaceutical industry’s grand challenge. Nat. Rev. Drug Discov. 9, 203–214 (2010).
- DiMasi, J. A., Grabowski, H. G. & Hansen, R. W. Innovation in the pharmaceutical industry: New estimates of R&D costs. J. Health Econ. 47, 20–33 (2016).
- Center for Drug Evaluation & Research. Drug Development & Approval Process. U.S. Food and Drug Administration https://www.fda.gov/drugs/development-approval-process-drugs (2019).