Alberto Galasso, University of Toronto, National Bureau of Economic Research and Centre for Economic Policy Research, and Hong Luo, Harvard Business School
Contact: a.galasso@rotman.utoronto.ca
Abstract
What is the message? Managing product liability risk is imperative for medical device firms. Effective risk management requires not only the development of a strategy to address liability claims at the litigation stage, but also at the product ideation and development stage. Technology safety features differ in their impacts on consumer willingness to pay and protection to liability litigation, and the development of risk-mitigating technologies involves multiple trade-offs that managers need to identify and consider.
What is the evidence? The findings are based on a review of the literature in management, law and economics, and industry case studies. The managerial implications are obtained through the development of a theoretical framework linking product liability and innovation strategies.
Timeline: Submitted: December 7, 2022; accepted after review: April 26, 2023.
Cite as: Alberto Galasso, Hong Luo. 2023. Managing Medical Device Liability through Innovation: A Strategic Approach. Health Management, Policy and Innovation (www.HMPI.org), Volume 8, Issue 1.
Product liability risk in the medical device industry
Product liability laws protect customers from defective and dangerous products. Effective liability systems not only compensate victims, but also act as an incentive to provide safer products. At the same time, product liability risk can expose firms to expensive litigation which can have detrimental impact on profitability and long-term reputation effects. For small firms, product liability litigation can be an existential threat.
Product liability cases have been increasing in the last decade, reaching about 60,000 filings in 2019 across U.S. courts. Medical devices and pharmaceutical products account for the majority of these cases, as firms in these industries took all the top 20 positions on the list of most active defendants. At the top of the list, Johnson & Johnson featured as defendant in 70,924 product liability cases during the 2015-19 period (Lex Machina, 2020). Many medical device liability cases were extensively covered by the media because of the pain and suffering those defective products caused, and the large damages awarded. Examples include litigation related to Bayer’s birth control devices, St. Jude’s implantable cardioverter-defibrillators, Stryker’s hip implants, and Covidien’s surgical staplers. Public attention to medical device litigation has also increased following the 2018 Netflix documentary ‘The Bleeding Edge.’
The importance of the issue implies that managing liability risk is imperative for medical device firms. Effective risk management requires not only the development of a strategy to address liability claims ‘ex-post’ at the litigation stage, but also ‘ex-ante’ at the product ideation and development stage. In other words, product liability risk should be an important driver of technology strategies and R&D investment decisions. In this article, we discuss several aspects of the relationships among product liability risk, safety, and innovation strategies of medical device companies.
Risk mitigating technologies
Risk mitigating technologies (RMTs) are innovations that reduce the probability of negative events or the severity of their consequences (Galasso and Luo, 2021). Drug containers with safety caps, for example, reduce the likelihood of children ingesting prescription drugs. Syringes with retractable needles prevent injuries and exposure to the contaminated needles. New types of hip implants producing less wear particles, or new silicone breast implants with lower risk of rupture are further examples of RMTs. RMTs may take various forms, depending on the nature of the hazards, the preferences of the consumers, and the technological possibilities. They can be incremental innovations that refine existing technologies or radical innovations that potentially establish entirely new classes of products.
An important difference between RMTs and technologies affecting other dimensions of quality (such as the image resolution of CT scanners or the look and feel of breast implants) is that the value created by RMTs is strongly influenced by non-market forces. Consider, for example, the development of a new CT scanner including dose display, alert and notification systems which can reduce the risk of over-radiation. The profits that an innovator can make will depend on the existing regulations on CT safety (will the device become a mandated safety feature?), on the liability system (how large are the damages in case of malfunctioning and over-radiation?) as well as on the media attention on the subject and on the level of consumer activism. Moreover, the efficiency of the insurance markets that can protect against the risk will also shape the market potential of RMTs.
From a business perspective, it is important to notice that RMTs generate value through two distinct, but related, channels. The first is consumers’ willingness to pay. RMTs lead to safer devices which generate benefits to patients and care-providers. Typically, a device which is safer than competing products in the market allows its producer to charge a price premium and obtain higher profit margins. The second mechanism by which RMTs create value is by reducing firms’ product liability costs. As safer devices are less likely to be involved in product liability lawsuits, RMTs reduce firm costs by decreasing the likelihood of costly litigation.
Innovation scholars often highlight the distinction between product and process innovation. The former includes technologies which improve the quality of existing products, the latter relates to reduction in firm operating costs. The discussion above indicates that RMTs inherently possess features of both product innovation (they increase consumer willingness to pay when consumer value safety) and process innovation (they reduce the expenses from product liability litigation).
A taxonomy of RMTs
Recognizing the two channels through which RMTs can generate value is important as it suggests that RMTs may differ across these dimensions. To understand the type of RMTs developed by a firm, managers should ask themselves a series of questions. First, they should consider whether the new technology generates higher consumer willingness to pay, and whether this can be leveraged by the firm to extract greater product market profits. Let us call this dimension the product market effect of the technology.
After considering the market potential of the technology, managers should assess its impact on product liability litigation. RMTs may reduce the exposure of firms to liability risk by reducing the likelihood of being involved in litigation, or by reducing the litigation costs and damages that firms expect to face if litigation takes place. Let us call this aspect the defensive effect of RMTs.
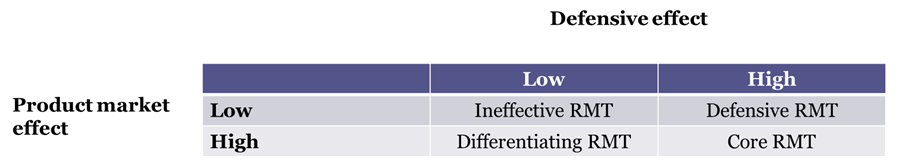
Figure 1 – A taxonomy of RMTs
Figure 1 exploits these differences to provide a taxonomy of RMTs depending on their product market and defensive effects. We define as ineffective RMTs those technologies which are not expected to have any traction with consumers and are not expected to improve the legal position of the firm in product liability suits. To make a business case for these RMTs is difficult and, in most cases, they should not be developed.
Defensive RMTs are innovations that can substantially improve a firm’s legal position, but that do not meaningfully increase the ability of firms to extract greater market profits. Childproof prescription drug containers are an example of defensive RMTs. These technologies, developed to make it harder for children to access and swallow medicines, can provide protection for pharmaceutical companies in product liability cases. For example, in 2018 the U.S. Department of Justice prosecuted a claim against the pharmaceutical firm Dr. Reddy’s Laboratories related to the distribution of prescription drugs in blister packs that were not child resistant. At the same time, survey evidence shows that many adults find these containers too hard to open. This is especially the case for the elderly and people with disabilities (Thwaites, 1999). In the context of diagnostic and therapeutic technologies, defensive RMTs include readiness check software, which prevents operators from using a device until a series of quality assurance checks are satisfied. These RMTs can substantially reduce the risk of equipment malfunction, and thus the liability exposure of the manufacturer. At the same time, clinicians’ willingness to pay for these features may be limited, as the software may slow down clinical workflow and reduce the number of patients seen by a practice.
Differentiating RMTs are technologies valued by customers, as they affect their perception of product safety, but do not generate substantial protection in the case of a liability suit. They create value through product market effects rather than defensive effects. These are typically new technologies that depart substantially from the more established technologies in the field. New robotic surgical systems are an example of differentiating RMTs. The robotic surgery industry has grown substantially since the FDA approval of Intuitive Surgical’s da Vinci system in 2000, with revenues reaching an annualized growth rate of 7.2% during the period 2015-19 (Crompton, 2020). Surgeons and hospital administrators report substantial safety benefits from robotic surgery. These include more precision during the operation, fewer post-operative complications, and shorter recovery time. At the same time, surgical robot companies have been the target of substantial product liability litigation. For example, our search of the litigation database LexMachina shows 253 federal district court product liability cases related to Intuitive Surgical between 2009 and 2022. The rapid evolution of robotic systems makes these producers easier targets of design defect lawsuits relative to other medical device firms which operate in fields where decades-old safety standards are in place. For example, in some of these cases plaintiffs allege that components in the arms of robotic systems can cause severe burns to healthy tissues and organs (Fox, 2013). More generally, the complexity and the novelty of these technologies make problems difficult to predict, and it is challenging to determine whether undesirable outcomes are caused by the surgeon, the design of the robotic device, or both.
Core RMTs are technologies that provide legal protection and high customer value in tandem. In other words, they generate value through both product market and defensive effects. Development of core RMTs can be a key driver of competitive advantage and firm growth. Take, for example, Becton Dickinson (BD) a medical device company perceived as a leader in product safety. A well-known set of products offered by BD are their syringes with retractable needles, pivoting needles and shielding needles, which reduce substantially the risk of exposure to blood borne pathogens such as HIV and hepatitis C for healthcare workers. In turn, this reduces product liability risk for the syringe manufacturer. BD market success from these devices led the firm to focus its entire technology strategy around healthcare worker safety (Pisano, 2019).
A second example of core RMTs is the introduction of iterative reconstruction (IR) in CT scanners. IR is an image construction technique which allowed for large levels of radiation dose reduction (up to 80-90 percent) in CT scanners relative to other imaging methods. The introduction of this RMT followed a series of investigations, prompted by media attention, on medical over-radiation accidents using imaging and radiation therapy devices. While these accidents were due mainly to misconfigurations by the hospitals, they led to greater demand for safer products and highlighted potential liability risk for CT scanner producers in the case of excessive radiation dosage. The industry reacted to these events launching new generations of CT scanners which featured IR and engaging in significant sales and marketing campaigns. For example, the front page of GE’s product brochure for Veo—one of their CT scanner using the IR algorithm—displayed the following message in large font: “The breakthrough that is rewriting the rule of CT imaging,” and adds that this technology helps physicians to achieve “the enhanced image quality at a radiation dose never before thought possible” (Galasso and Luo, 2021).
Many medical devices are purchased and used by hospitals, rather than directly by patients. When this is the case, RMTs may reduce product liability litigation exposure both for device manufacturers and for clinical users. For example, an RMT reducing CT scanner over-radiation risk may substantially decrease the litigation exposure of the hospitals, which increases their willingness to pay for the device. The same RMT also reduces the litigation exposure for the manufacturer, which decreases its operating costs. This implies that, in some cases, litigation motives alone may suffice to generate a core RMT.
Implications for technology strategy
The RMT taxonomy discussed above highlights the key features that technology managers must consider when assessing the desirability of new medical innovation related to device safety features. The analysis has several implications for medical device companies.
- Safety technologies generate multiple trade-offs. Whether or not to commercialize a new technology is a key strategic decision that has a long-term impact and is difficult to reverse. It is thus crucial to price correctly the factors affecting technology development strategies. Our framework indicates that the development of RMTs involve multiple trade-offs that managers need to identify and consider. The first set of factors involves the possible impacts that enhanced device safety may have on other quality dimensions. For example, new models of CT scanners featuring lower levels of radiation may perform worse on other quality dimensions such as speed and image clarity. The overall willingness to pay of consumers – its product market effect – will depend on the combination of these different aspects. The second potential trade-off involves possible tensions between the product market and the defensive effects of the technology. In some cases, large reductions in litigation risk in a market may be associated with lower willingness to pay for the product. For example, Galasso and Luo (2017) provide evidence supporting the idea that tort reforms reduce physicians’ propensity to adopt technologies that reduce the probability of malpractice disputes. In others, higher willingness to pay for safety features may also be accompanied by greater risk of product liability litigation. Mispricing these factors can undermine the technology development analysis.
- The value of safety features evolves over time. The incentives to develop and commercialize RMTs can shift over time and may be shaped by changes in the business and social environment in which a device company operates. For example, media coverages of accidents and new technologies can play a crucial role in disseminating information on health hazards to the general public, thereby influencing the demand for safety. As documented by Galasso and Luo (2021), the development of several RMTs for CT scanners was spurred by a series of effective investigative reporting by The New York Times. Similarly, changes in consumer activism and education may lead to greater risk of liability litigation, which in turn affects the incentive to develop RMTs.
- Safety education can complement technology development. In the case of defensive RMTs, a firm may consider complementing the commercialization of the technology with an information campaign in which the hazards are made more salient to customers. For example, Johnson & Johnson, a technology leader in child-proof containers, is also a founding sponsor of ‘Safe Kids Worldwide,’ a nonprofit organization focused on preventing injuries in children that has developed information campaigns on safe medicine storage. These education programs provide value to the population and can shape user preferences, mitigating the trade-off between safety and other quality aspects of the products.
- The regulatory and legal environment affects safety technological advances. For many areas of medical technologies, such as robotic surgery and devices using artificial intelligence, governments are questioning whether and to what extent the current legal framework on safety and liability can adequately protect consumers. In February 2020, the European Commission released its “Report on the safety and liability implications of Artificial Intelligence, the Internet of Things and Robotics,” suggesting that there are important gaps that policy makers need to address to ensure consumer protection and to incentivize innovation. Such legislative uncertainty may reduce the value of RMT development, as firms may prefer to wait for greater clarity in the regulatory landscape. In some cases, firms may even be concerned about the possibility that the entire product category will be banned. For example, the FDA banned the cosmetic use of silicone breast implants in 1992 (for 14 years) due to health concerns of leaking silicone (Angell, 1996). This suggests that medical device firms may benefit from taking a more proactive role toward legislators, especially when developing differentiating RMTs in new and rapidly growing fields such as devices exploiting artificial intelligence and robotics. This can be done through direct advocacy or through participation in industry associations.
- The industry structure affects the incentives to develop RMTs. Changes in consumer risk perception often go beyond a particular product or manufacturer and spill over to similar or related products. Galasso and Luo (2021) show that media coverage of over-radiation accidents led to industry-wide changes in R&D strategy both from firms directly involved in the accidents, as well as from firms that were not. The largest manufacturers had a crucial role in developing and introducing new RMTs and applied for more patents related to radiation safety. This can be explained by economic incentives as large market shares provide greater incentives to internalize the liability and reputation costs. Moreover, large firms often have an advantage in appropriating returns from investments in safety across a wide range of products and customer segments. Agreement over new safety standards is also easier to reach in industries where the number of players is not too large.
A common view in academic and policy debates is that product liability risk retards innovation. For example, Porter (1990) recommends “a systematic overhaul of the U.S. product liability system,” arguing that in the United States, “product liability is so extreme and uncertain as to retard innovation.” While this can be the case in some circumstances (see Galasso and Luo, 2022), the framework presented in this paper suggests that the link between liability and innovation is complex and nuanced. Firms may respond to product liability risk through the development of RMTs. In practice, technology safety features differ in their impacts on consumer willingness to pay and protection to liability litigation. The development of RMTs might not be the right approach for every company, but in several situations medical device manufacturers can benefit from it. The key to success is to recognize the role of technology safety in innovation strategies, and to carefully consider its product market and defensive effects.
References
Angell, Marcia (1996) Science on Trial: The Clash of Medical Evidence and the Law in the Breast Implant Case W. W. Norton & Company
Crompton, Thomas (2020) “Robotic Surgery Equipment Manufacturing” IBISWorld industry report OD4074
Fox, Maggie (2013) “Electrical burns may burst surgical robot’s bubble” NBC news online article available at https://www.nbcnews.com/healthmain/electrical-burns-may-burst-surgical-robots-bubble-6C10321766
Galasso, Alberto, and Hong Luo (2017) “Tort reform and innovation,” Journal of Law and Economics 60: 385-412
Galasso, Alberto, and Hong Luo (2021) “Risk-mitigating technologies: The case of radiation diagnostic devices” Management Science 67: 3022-3040
Galasso, Alberto and Hong Luo (2022) “When does Product Liability Risk Chill Innovation? Evidence from Medical Implants,” American Economic Journal: Economic Policy 14: 366–401
Lex Machina (2020) Product Liability Litigation Report
Pisano, Gary (2019) Creative construction: The DNA of sustained innovation. Public Affairs
Porter, Michael (1990) The competitive advantage of nations, Free Press, New York
Thwaites, John (1999) “Practical aspects of drug treatment in elderly patients with mobility problems,” Drugs and Aging 14: 105-114