Cheyenne Ariana Erika Modina, Sandra Waugh Ruggles, Juliana Perl, and Josh Makower, Stanford Byers Center for Biodesign, School of Medicine, Stanford University
Contact: jmakower@stanford.edu
What is the message? For medical innovators, obtaining a Category I Current Procedural Terminology (CPT) code from the American Medical Association (AMA) has become fundamental to unlocking reimbursement and patient access to a medical product, procedure or service in the United States. For those with novel innovations requiring assignment of a new code, the significant challenges posed to patient access by the current criteria could be improved with a more transparent, predictable, and achievable process, so that healthcare innovation and patient access to FDA-cleared and clinically proven therapies can flourish.
What is the evidence? A survey of different stakeholder groups with personal knowledge of the information used to apply for and achieve a Category I CPT code for a new treatment or diagnostic technology was designed and analyzed to assess the current challenges to traverse the CPT process, and understand the reasons for current Category I code challenges and their potential impact on the future of healthtech innovation.
Timeline: Submitted: April 3, 2024; accepted after review April 7, 2024.
Cite as: Cheyenne Ariana Erika Modina, Sandra Waugh Ruggles, Juliana Perl, Josh Makower. 2024. Current Common Procedural Terminology (CPT®) Coding Process Challenges: Impact on the HealthTech Innovation Ecosystem. Health Management, Policy and Innovation (www.HMPI.org), Volume 9, Issue 1.
Introduction
Established in 1966 and maintained by the American Medical Association (AMA), Current Procedural Terminology (CPT) is a uniform language of descriptive terms and identifying codes used to communicate across healthcare, enabling processing of insurance claims and advanced analytics for medical, surgical, and diagnostic procedures used to advance patient care. Updated annually, CPT has evolved over more than 50 years as medicine has advanced to incorporate new practices, paradigms, and applications including electronic health records, precision medicine, the COVID-19 pandemic, genomics, digital medicine, and augmented intelligence (AI)-powered medical service.
There are three categories of CPT codes. Category I (CAT I) CPT codes, released on January 1 each year by AMA, are restricted to clinically recognized and generally accepted services. These five-digit codes have descriptors that correspond to a procedure or service. In general, a proposed CAT I descriptor needs to be unique, well-defined and describe a procedure or service which is clearly identified and distinguished from existing procedures and services already in CPT. In addition to meeting specific general criteria, a proposal for a new or revised CAT I code must satisfy all of the following criteria:1
- All devices and drugs necessary for performance of the procedure or service have received FDA clearance or approval when such is required for performance of the procedure or service.
- The procedure or service is performed by many physicians or other qualified healthcare professionals across the United States.
- The procedure or service is performed with frequency consistent with the intended clinical use (i.e., a service for a common condition should have high volume).
- The procedure or service is consistent with current medical practice.
- The clinical efficacy of the procedure or service is documented in literature that meets the requirements set forth in the CPT code-change application.
The criteria that procedures or services must be performed by many physicians or other qualified healthcare professionals nationwide, with frequency consistent with the intended clinical use, are collectively and informally referred to throughout this paper and in industry as the “widespread use” criteria.
Importantly for physicians, patients and innovators, CAT I CPT codes are priced on the Medicare Physician Fee Schedule and are generally more favorably reviewed for coverage by Medicare and commercial payers.
Category II (CAT II) CPT codes are optional, supplemental tracking codes to CAT I codes that describe performance and measurement, purposed for collecting information on the quality of care and thus reducing administrative burdens on healthcare professionals.2
Category III (CAT III) CPT codes were introduced by AMA in 2001 as a temporary code to enable providers and health systems to properly document and report data regarding new and emerging technologies, services, and procedures. They are also used to substantiate CAT I code criteria such as “widespread use,” and to track product utilization in Category B clinical studies.3 Although obtaining a CAT III code does not require FDA approval or clearance, nor published peer-reviewed evidence, as CAT I codes do, other criteria need to be met including that the procedure or service is currently or has recently been performed in humans.2 The AMA releases new CAT III codes twice a year, and the codes usually remain active for five years. Some but not all CAT III codes convert to CAT I.
The entire CPT code set is updated at least annually through a publicly accessible process by the CPT Editorial Panel. The Panel’s 21 members include nominated physicians, healthcare professionals who are experts in their medical specialty, and others representing private insurance companies, hospitals, and other interested parties. All complete CPT code change applications are reviewed and evaluated by CPT staff, the CPT/HCPAC (Health Care Professionals Advisory Committee) and the CPT Editorial Panel. Additionally, the CPT Advisory Committee, comprised of representative physicians selected by the national medical specialty societies from the AMA House of Delegates and the HCPAC, serves as an expert resource to the Editorial Panel. The Panel can choose to: add a new code or revise existing nomenclature; refer the agenda item to a workgroup for further study; postpone to a future meeting to allow for additional information to be submitted; or reject the item. Panel convenings happen three times per year to ensure timely updates on clinical practices and the latest innovations.
The pace of CPT code growth is increasing over time, with more than 1,200 (net) new codes added in the last decade. The CPT 2024 code set, released by AMA in September 2023, created 349 editorial changes, including 230 additions, 49 deletions, and 70 revisions.4 When the inaugural data set was released, 3,554 codes were included, and today there are 11,163 codes describing the medical procedures and services available to patients. In parallel with the pace of medical innovation, the use of CAT III codes has expanded rapidly in recent years, up 246% since 2011, according to AMA.5
Following assignment of a new CAT I CPT code, the AMA Relative Value Scale Update Committee (RUC) reviews and advises the Centers for Medicare and Medicaid Services (CMS) on the relative values of each code. Subsequently, CMS establishes the payment for each CPT code in the Medicare Physician Fee Schedule and the Prospective Payment Systems.6 The entire new CPT code application process can take from 18 to 24 months. Moreover, it may take two to five years to collect all the literature and evidence that meet criteria.2 Failure to obtain a CPT CAT I code can delay market access for a healthtech product by as long as 18 months (e.g., the September 2024 CPT Editorial Panel meeting is the last opportunity to secure a CAT I code for 2026). Meeting the CPT code requirements and achieving the assignment of a CAT I code have become fundamental to commercialization because alternative coding strategies (eg: unlisted codes and CAT III codes) do not have assigned facility and/or physician payment amounts. Appropriate codes and payment, along with insurance coverage, are needed to ensure patient access.
We examine the impact of the CAT I criteria of “widespread use” on perceptions of the new code request process, and profile the climate of novel product introduction when a code is not available. The objective of this research is to assess the current challenges for innovators to traverse the AMA CPT process. It aims to understand the reasons for current CAT I CPT code challenges and their potential impact on the future of healthtech innovation.
Study Data and Methodology
Survey Development: An anonymous survey was developed and coded using Qualtrics XM (Qualtrics Version January 2024, Provo, UT)[*].7 It was divided into two sections: demographic data to screen respondents and survey questions focusing on current challenges (see Appendix 1: Survey). The inclusion criteria are as follows:
- Must be an investor, innovator, entrepreneur, senior leader with general management authority, clinical, health economics and outcomes research (HEOR)/market access professional, physician, or an area expert (such as a reimbursement consultant).
- Must be personally knowledgeable of the information used to apply for and achieve a CAT I CPT code for a new treatment or diagnostic technology.
Data Collection: The survey was deployed via email through the Stanford Biodesign network and the membership lists of Advanced Medical Technology Association (AdvaMed) and the Medical Device Manufacturers Association (MDMA) in January 2024. The posting had the specific request that only those with experience with the CPT process click the link to attempt the survey. A follow-up email was sent three days later to attract additional respondents. Data collection continued for a total of six days.
A total of 333 respondents clicked on the survey link, of whom 272 completed the survey, giving a click-through to completion rate of 81.6%. To avoid duplicates, each IP address was checked to ensure there were no repeat respondents. The respondents then answered a series of questions to reflect the inclusion criteria above. Out of the 272 respondents, only 174 passed the inclusion criteria.
Data Cleaning and Analysis: Results were exported from Qualtrics XM platform (Version January 2024)[*]7 to Microsoft Excel Version 16.838 and NVivo Release 1.7.19 for data cleaning and analysis. Only one project member had access to the full results to de-identify responses to open-ended questions that could reveal personal information about respondents. After de-identification, descriptive statistical analyses were conducted using Microsoft Excel, while qualitative analyses, including word frequency and thematic analyses, were performed in NVivo.
Inductive thematic analysis was used to identify the themes within the qualitative data. One reviewer examined the transcription, and then, using the preliminary data from word frequency as a guide, generated initial codes. After this initial coding, similar codes were grouped together to form initial themes. The reviewer then revisited the transcript to finalize the themes.
Study Results
Characteristics of Respondents
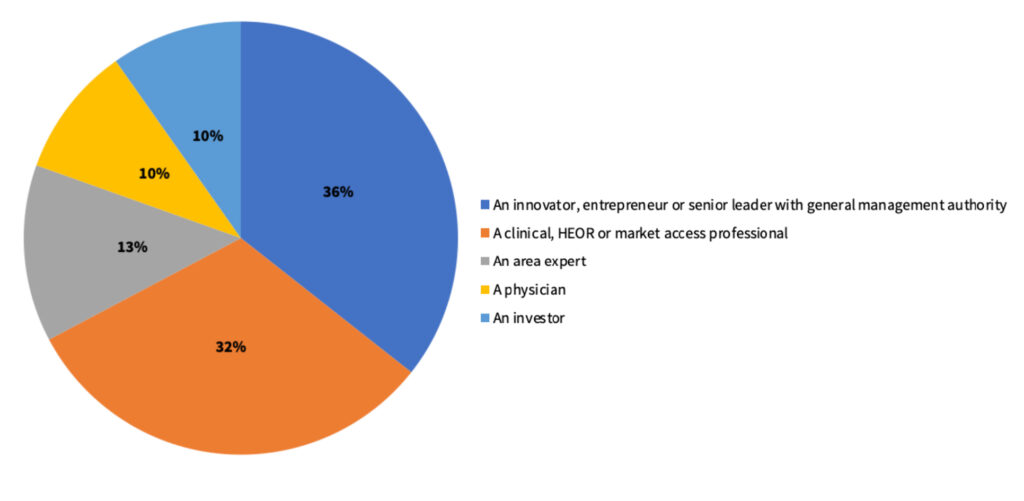
Out of the 272 innovators and investors who answered the survey, 64% of the respondents (n=174) were sub-selected as they were knowledgeable about the CAT I code process for new treatment or diagnostic technologies.
Out of the 174 final respondents, 36% were innovators, entrepreneurs, or senior leaders with general management activity. This was followed by clinical/health economics and outcomes research (HEOR)/market access professionals (32%), area experts (13%), physicians (10%), and investors (~10%).
Figure 1. Distribution of survey respondents based on occupation.
Challenges with the Current CAT I Processes
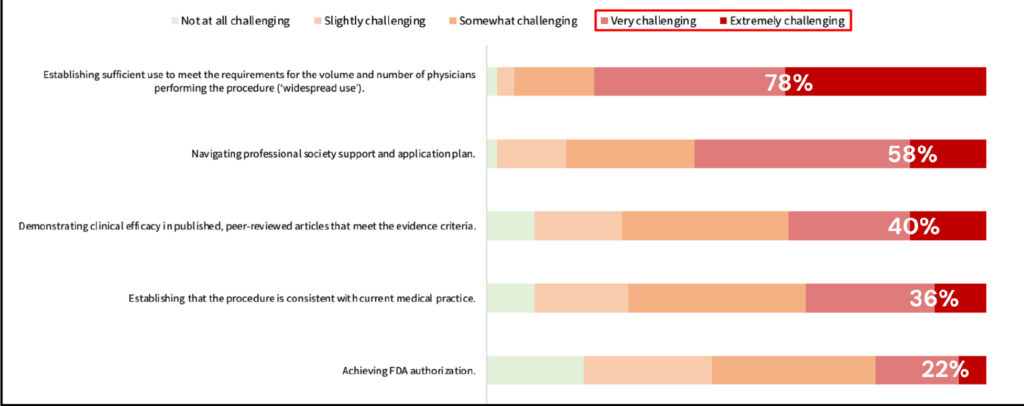
Respondents were asked, “How challenging is it to meet the typical steps in the process of obtaining a CAT I code?” Among the various steps, achieving “widespread use” emerged as the most challenging, with 78% of respondents rating it as very challenging to extremely challenging. Navigating professional society support and application plan was identified as the next most challenging step, with approximately 58% of respondents experiencing difficulty in this process. Demonstrating clinical efficacy in published, peer-reviewed articles that meet the evidence criteria and establishing that the procedure is consistent with current medical practice were very challenging and extremely challenging for 40% and 36% of respondents, respectively. The least challenging step was found to be obtaining FDA authorization, with only 22% of respondents rating it as very or extremely challenging.
Figure 2. Ranked typical steps to obtain CAT I code from most to least challenging.
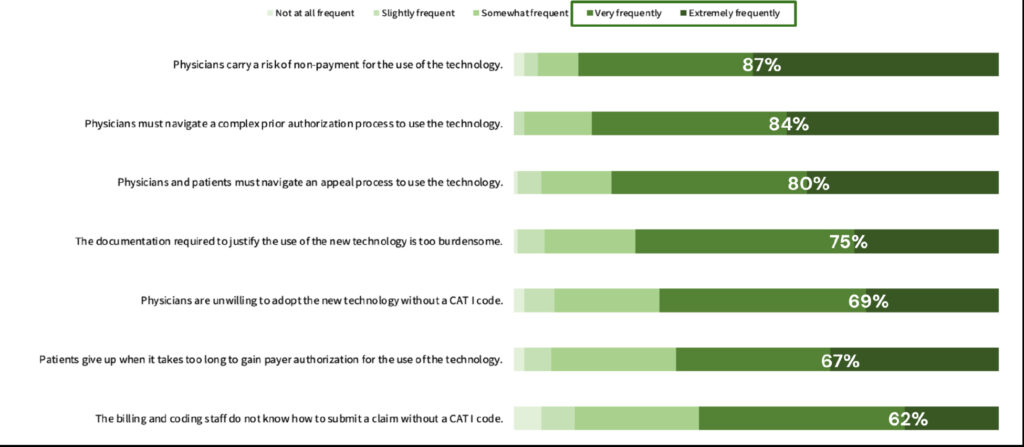
When asked to rate the frequency of objections to using a new technology before the effective date of a CAT I code, respondents identified the top two primary concerns: “physicians face the risk of non-payment for using the technology” and “physicians must navigate a complex prior authorization process to use the technology.” Roughly 87% and 84%, respectively, rated these concerns as occurring very frequently or extremely frequently (Figure 3). The least frequent objection by 62% of respondents was that “billing and coding staff are unsure how to submit a claim without a CAT I code.”
Figure 3. Ranked frequency of the following objections to the use of a new technology prior to the effective date of a CAT I code from most to least frequent.
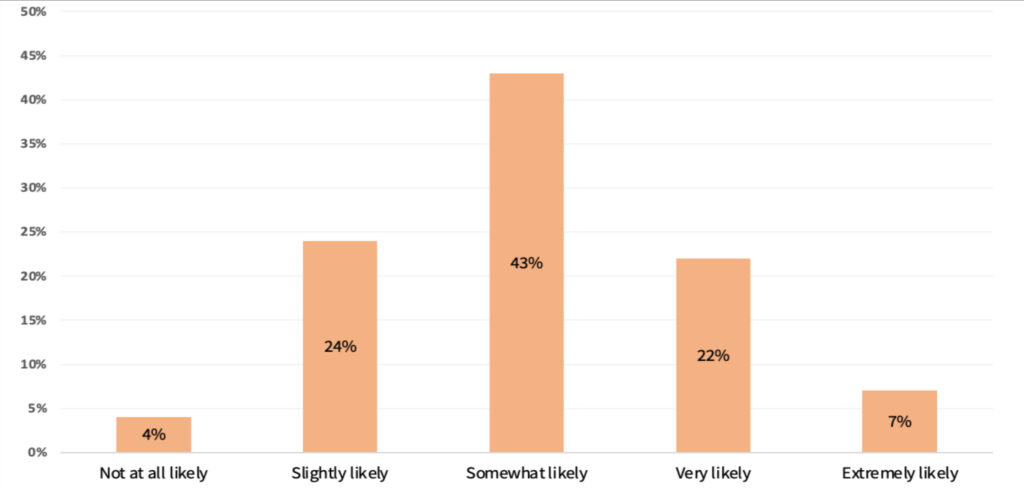
Around 43% of the respondents indicated that they would somewhat likely initiate a new project addressing a compelling unmet clinical need even if it requires obtaining a new CAT I code. Similarly, 29% indicated they are still either very likely or extremely likely to pursue such an opportunity. In contrast, 28% answered slightly likely to not at all likely.
Figure 4. Likelihood to address an unmet clinical need requiring a new CAT I code.
Other Challenges with the CAT I Code Process
Several themes emerged from the responses regarding challenges associated with the CAT I process. These themes, as highlighted in the survey, emphasize the impact of such challenges on innovation—especially for small companies or startups with novel technology — and on patient care, as respondents highlighted how these challenges create barriers to accessing interventions.
Challenges in Demonstrating “Widespread Use” and Gathering Evidence
Similar to the results in Figure 2, and reflecting an overall theme of the survey results, the criteria of “widespread use” has been challenging for innovators to meet. “The additional requirement of demonstrating ‘widespread use’ creates a barrier to patient access,” commented one respondent, especially for emerging technologies as “the burden of evidence is overwhelming to have one Level 1 clinical trial and one Level 2 clinical study of non-overlapping patient populations.” Aside from that, respondents mentioned that “widespread use” is not clearly defined. Respondents suggested that widespread use should not be contingent on creating a code for a viable procedure.
High Costs and Resources Required for Obtaining CAT I Code
Respondents voiced that applying for new codes for novel technologies is “arduous, time consuming and capital intensive, especially for small novel companies.” One innovator recalled that it took the company “five years and roughly $10 million for prospective and comparative trials” to manage the process. Another innovator mentioned that it is an additional six to eight years for these technologies to have an existing CPT code. One respondent pointed out, “most startups don’t have these resources,” and that this is an obstacle to adoption of innovative and sometimes life-saving technology.
Lack of Transparency Throughout the Process
Respondents reported the CPT process to be inconsistent, opaque, and lacking in transparency. According to them, the inconsistency arises from the manner in which the CPT Editorial Panel reviews applications, especially in regard to “widespread use.” This results in an opaque process for which the information sources available online “do not necessarily reflect the best practice for obtaining a code.” This results in innovators expending valuable resources, for example hiring an outside market access firm to help guide them through the complexities of the CPT process.
Other respondents have pointed out the difficulty in shifting from CAT III codes to CAT I codes. Respondents cited that CAT III codes are reportedly “still non-covered by most payers” while “some have blanket policies against coverage.” They reported that this, together with the lack of transparency within the entire process, highlights the systemic issues that innovators face.
After a CAT I code has been granted, respondents reported issues with the RUC (AMA/Specialty Society Relative Value Scale Update Committee) process. In addition to the challenge of obtaining a CAT I code, an innovator mentioned that the RUC process can be very “inhibitive to new technology” and lacks transparency. Respondents expressed concerns that this is due to reliance on outdated methodologies, leading to continuous challenges for innovators even after securing a CAT I code.
Lack of Support from Medical Societies and Specialty Groups
Another recurring theme in the survey was the lack of support from medical societies and specialty groups. A respondent mentioned that there is “no willingness to meet with industry to have constructive and creative discussions about supporting market access for new technology.” Respondents wish for a more transparent and objective process, especially when gaining specialty society support. Engaging with the CPT staff of medical societies is initially easy, respondents commented, but the coalition of societies that “need to agree on clinical studies required over and above the established CPT requirements” has become one of the most challenging issues for innovators.
Impact on Innovation and Patient Care
Despite companies following the required processes for FDA approval and CAT I code creation, innovators have voiced that they still encounter an “uphill climb” for payment that can often lead to very limited patient access and company failure. Multiple respondents have mentioned that they have been discouraged with the monumental challenges involved in obtaining a CAT I code. Given the high costs associated with obtaining a new code, innovators stated that they can be left with no choice but to integrate such costs into the selling prices, and as a result, “raising the cost to patients and the healthcare system.”
These ongoing challenges hinder the development of novel concepts that support physicians “who seek to create a better patient outcome above all else,” stated one respondent, signaling that they impact overall innovation and patient care. Respondents mentioned the importance of having this stringent coding process in place, but “achieving a code is a very onerous process.” Ultimately, this process impacts access to innovation for both physicians and patients.
Suggestions for Change
Respondents suggested solutions including societies appointing a designated, full-time expert physician for coding to streamline the process and facilitate collaboration with industry. Additionally, they recommended the creation of a quality system to track new CPT code requests, and standardizing responses to queries and requests from the industry across societies.
Discussion
The survey data emphasize the significant hurdle posed to patient access by the complex, expensive nature of pursuing a new CAT I code for a medical technology. Qualitative themes described current challenges innovators are facing, including demonstrating “widespread use,” high costs and resources, lack of transparency, and insufficient support from physician societies for companies initiating the process. To address these issues, respondents suggest increased transparency and cooperation from societies in establishing CAT I codes.
Societies, however, have also pointed out the challenges in the coding processes that they are encountering. Cathleen Biga, MSN, RN, vice president of non-profit medical society, the American College of Cardiology (ACC), has said that “the system is broken,” and identified the RUC process as a problem. Biga then argues that new technologies play a big role in lowering healthcare costs and improving patient outcomes, but patients cannot benefit from these advances unless insurance and Medicare pays for them. She added that changes in payments are very slow for new technologies that impact patients. Her position aligns with the survey results, as the top two primary objections in using a new technology before the effective date of a CAT I code are “physicians face the risk of non-payment for using the technology” and “physicians must navigate a complex prior authorization process to use the technology.”10
There are also a number of ways in which individual physicians play critical roles in coding. They ensure that the technology is consistent with current medical practices and aid in providing evidence to meet the requirements in processing a CPT code. Physicians are also directly involved in the AMA CPT Editorial Panel, where they are either nominated to be a part of the panel, or work with societies to send proposals for which technologies should have codes.11 Additionally, as a significant part of the process of obtaining a CAT I code, physicians and other healthcare professionals perform the specific procedures or services.11
The volume requirement is usually achieved by physicians going at risk of non-payment in order to support the “widespread use” criteria, which in itself places an economic hurdle on every physician wishing to advance new technology for patient care. This is further compounded by the fact that the actual numerical formula or threshold for clearing the criteria has remained opaque over all these years. This vague and seemingly subjective requirement can present a major hurdle to commercialization, because without a CAT I code, many payers will not reimburse for a product or service.
This creates a “chicken and the egg” situation, in which physicians are hesitant to perform new procedures when, as shown in Figure 3, there are uncertain payment prospects or prior authorization requirements. These uncertainties and complexities have the potential to be solved if a CAT I code were in place. However, such a solution can only be facilitated by widespread uptake of the technology. The result is a stalemate, in which clinicians cannot reliably perform new procedures and no progress is made towards a new code.
The most unfortunate outcome of this process is the resulting limited patient access. Central to a clinician’s payment and operations concerns is a patient who could stand to benefit from a new technology that has already received FDA authorization for patient use. Without coding, clinicians are often unwilling to offer a procedure—leaving a supposedly commercially available technology just out of reach. For clinicians willing to take the risk, their practice, hospital, or patient may be left with large bills for an uncovered procedure.
These challenges to obtaining new CPT codes are well known throughout the medical technology ecosystem, with a previous paper citing the reimbursement pathway as the most important external factor for investment decisions.12 As a result, early risk-analyses for company development may drive the innovation ecosystem as a whole to avoid pursuing new technologies that would not fall under currently available CPT codes. As a consequence, this prevents companies and investors from developing the most novel of technologies that are not categorized through previously established procedures.
Recommendations
The AMA, as the nation’s largest medical association with more than 271,000 members, states that its purpose is “to promote the science and art of medicine and the betterment of public health.” The association also states that it delivers on this mission by representing physicians with a unified voice in courts and legislative bodies across the nation, removing obstacles that interfere with patient care, leading the charge to prevent chronic disease and confront public health crises, and driving the future of medicine to tackle the biggest challenges in healthcare and training the leaders of tomorrow.13
One recent example of the AMA’s commitment to drive the future of medicine and address the needs of providers and patients in a rapidly evolving healthcare environment is its Digital Medicine Payment Advisory Group (DMPAG), initiated in late 2016.14 Over the past few years, DMPAG has achieved a significant and measurable impact on digital medicine intervention adoption by introducing CPT codes for remote physiologic monitoring, remote therapeutic monitoring, AI and other digital innovations. The AMA is also focused on educating members about new innovations and emerging trends in healthcare. But, as stakeholder respondents to the current survey have made loud and clear, more needs to be done to support innovation.
Other agencies integral to innovators’ success, FDA and CMS are doing their part to improve pathways to foster medical innovation and more expediently bring technologies into the hands of healthcare providers and patients. This includes FDA’s Breakthrough Devices Program, with updated final guidance issued in September 2023, intended to provide patients and providers with timely access to medical devices by speeding up development, assessment, and review for premarket approval, 510(k) clearance, and De Novo marketing authorization.15 In parallel, CMS issued a proposed procedural notice outlining a new Medicare coverage pathway to achieve more timely and predictable access to new medical technologies for people on Medicare.16 The Transitional Coverage for Emerging Technologies (TCET) pathway for Breakthrough Devices supports both improved patient care and innovation by providing a clear, transparent, and consistent coverage process while maintaining robust safeguards for the Medicare population.17 In another example, in large part due to strong advocacy efforts by AMA, physicians, and patients, CMS is reforming the costly and inefficient prior authorization process (cited by survey respondents as an issue; see Figure 3) under a newly issued final rule. The new rule will reduce patient care delays and the administrative burdens long shouldered by physicians, and save practices an estimated $15 billion over the next decade, according to CMS.18
Noting the significant strides being made across the medical regulatory and reimbursement ecosystem to improve access to medical innovation and advance patient care, and respondents’ opinions revealed in the current survey, we recommend that AMA policymakers take steps to devote resources to collaborating with innovators, physicians and other healthcare stakeholders to refine and reimagine the “widespread use” criteria—and consider fine-tuning the policy so that specific levels of use are considered—to more appropriately fit the realities of novel medical product and service development.
Innovators themselves can also play an impactful role in planning ahead for their reimbursement strategy and engagement with professional societies for CAT I CPT code coverage. It is essential that innovators develop and execute a robust plan in support of an eventual CAT I code application and approval. This could include partnering with physicians and other thought leaders to design robust clinical studies and appropriate diagnostic and treatment criteria, and ensuring sponsored clinical studies are submitted for peer-reviewed publication. Innovators can familiarize themselves with the process for a CPT code application through the extensive materials on the AMA website and learn about the CPT code decision-making process by attending the public meetings that are held multiple times per year.19
For medical innovators, the process of applying for and achieving a CAT I CPT code is a challenging and costly process that has become fundamental to unlocking reimbursement and patient access in the United States. The CAT I requirement of “widespread use,” in particular, has become a roadblock for innovators with novel products or services and the physicians that use them, that is having a negative impact on the innovation ecosystem and patient care. There is a strong and near-term need for a more transparent, predictable, and achievable CAT I CPT code process to ensure that healthcare innovation and patient access to FDA-cleared and clinically proven therapies can flourish and be preserved for future generations. Ultimately, it is critical that all stakeholders—AMA policymakers, innovators, physicians, and other healthcare providers—collaborate closely to ensure these recommendations are translated into tangible benefits for patient care. By doing so, this will allow for the continuous advancement of medical practices and technologies, for access to cutting-edge medical care and better patient outcomes.
Study Strengths and Limitations
Among the strengths of the current analysis is its reliance on data from a cross-section of healthcare innovation professionals who are personally knowledgeable of the information used to apply for and achieve a CAT I CPT code for a new treatment or diagnostic technology, and thus are qualified to provide an informed opinion of the process.
However, this research is also subject to several limitations. There is potential response bias in the respondent group as the survey was distributed only to the Stanford Biodesign network and the AdvaMed and Medical Device Manufacturers Association membership lists, that together represent a majority, but not all healthtech innovators in the United States. Also, previous research notes that an inherent weakness to surveys is that high-quality survey results come from participants who are motivated to optimize the response process, and who have a desire for self-expression, intellectual challenge, and a desire to be helpful.20
In addition, some respondents’ answers may be biased based on their subjective positive or negative experience with the CPT code process, and the direct impact on the company they were or are associated with that applied for a CAT I CPT code. Additionally, being that the survey included only five questions, its conclusions are limited to the key challenges revealed in this analysis, and in the open-ended feedback provided by respondents.
As there is a lack of existing research on the specific topic of CPT Code process challenges and impact on the healthtech innovation ecosystem, and the conclusions of the current survey point to compelling challenges with the current CAT I process, there is ample future opportunity to elucidate additional valuable insight and recommendations from the different stakeholder groups involved in applying for and achieving a Category I CPT code for a new treatment or diagnostic technology. These future investigations can invite a broader discussion of the critical issues impacting reimbursement and adoption of novel technologies.
Conclusion
The high evidentiary standards that the AMA has established for CPT CAT I criteria are indeed appropriate when assessing novel procedures or services intended for patient use. These standards include the need for well-documented clinical efficacy data, FDA authorization, and consistency with current medical practice. However, it is also clear that the challenges involved in obtaining a CPT CAT I code—specifically, achieving “widespread use”—are negatively impacting the innovation ecosystem and patient care. This discourages some innovators from pursuing innovations that might require a new CPT CAT I code in the future. The subsequent impact of innovators turning away from advancing important new medical innovations due to the challenges of this process could significantly affect patient care and access.
While considering alternatives to CPT’s “widespread use” criteria, it is also important to recognize that there may be some potential drawbacks of removing or modifying the criterion for novel technologies completely. Doing so may result in the establishment of additional codes at a greater rate, which may increase the workload and turnaround time for decisions made by the editorial panel. It is also possible that some procedures may obtain a CPT I code but may remain less utilized. Overall, however, these potential concerns do not substantially overshadow the data reported from this survey which calls into question the utility or reasonableness of setting “widespread use” as a criterion for novel technologies and suggests that improvements and adjustments should be made to ensure that the CPT CAT I process continues to move in time with innovations in medicine.
Considering the fragile state of the health technology innovation ecosystem, along with the rapid pace of scientific progress and medical innovation aimed at addressing urgent unmet clinical needs and improving the quality of life of patients, there is a strong and immediate need for a more transparent, predictable, and achievable CPT CAT I code process. Such improvements are crucial to ensure that healthcare innovation flourishes and that patient access to FDA-cleared and clinically proven therapies is preserved for future generations.
Notes
[*] The survey design and data collection for this paper was generated using Qualtrics software, Version January 2024 of Qualtrics. Copyright © 2024 Qualtrics. Qualtrics and all other Qualtrics product or service names are registered trademarks or trademarks of Qualtrics, Provo, UT, USA. https://www.qualtrics.com
References
- Criteria for CPT® Category I and Category III codes. American Medical Association. Published September 22, 2023. Accessed March 22, 2024. https://www.ama-assn.org/practice-management/cpt/criteria-cpt-category-i-and-category-iii-codes
- Kuo TY, Manaker S. Reimbursement Strategies and CPT Codes for Device Development. Academic Entrepreneurship for Medical and Health Scientists. Published online April 16, 2021. doi:10.21428/b2e239dc.8e3cdecb
- Leslie-Mazwi TM, Bello JA, Tu R, et al. Current Procedural Terminology: History, Structure, and Relationship to Valuation for the Neuroradiologist. AJNR Am J Neuroradiol. 2016;37(11):1972-1976. doi:10.3174/ajnr.A4863
- AMA releases the CPT 2024 code set. American Medical Association. Published September 8, 2023. Accessed March 22, 2024. https://www.ama-assn.org/press-center/press-releases/ama-releases-cpt-2024-code-set
- Stanford Byers Center for Biodesign, Fogarty Innovation. CPT®: The Language of Medicine for Innovators. Stanford Byers Center for Biodesign. Accessed March 22, 2024. https://biodesign.stanford.edu/programs/policy-program/publications-testimony-events.html
- Medicare Payment Systems. Accessed April 17, 2024. https://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNProducts/html/medicare-payment-systems.html
- QualtricsXM. Qualtrics Version January 2024. Published online 2024. https://www.qualtrics.com.
- Microsoft. Microsoft Excel. Published online 2024.
- QSR International. NVivo. Published online 2024. https://www.qsrinternational.com/nvivo-qualitative-data-analysis-software/home
- Fornell D. Reimbursement challenges raising concerns in cardiology. Cardiovascular Business. Published March 21, 2023. Accessed March 28, 2024. https://cardiovascularbusiness.com/topics/healthcare-management/healthcare-economics/medical-billing-and-coding/reimbursement-challenges-raising-concerns-cardiology
- Finch M. Reimbursement Basics. Published online November 10, 2022. Accessed March 22, 2024. https://pressbooks.umn.edu/mdih/chapter/reimbursement-basics/
- Ruggles SW, Perl J, Sexton Z, Schulman K, Makower J. The Need for Accelerated Medicare Coverage of Innovative Technologies: Impact on Patient Access and the Innovation Ecosystem. HMPI. Published January 17, 2022. Accessed March 22, 2024. https://hmpi.org/2022/01/17/the-need-for-accelerated-medicare-coverage-of-innovative-technologies-impact-on-patient-access-and-the-innovation-ecosystem/
- About the AMA. American Medical Association. Accessed April 16, 2024. https://www.ama-assn.org/about
- Digital Medicine Payment Advisory Group. American Medical Association. Published March 20, 2024. Accessed March 22, 2024. https://www.ama-assn.org/practice-management/digital/digital-medicine-payment-advisory-group
- Health C for D and R. Breakthrough Devices Program. FDA. Published online March 20, 2024. Accessed March 22, 2024. https://www.fda.gov/medical-devices/how-study-and-market-your-device/breakthrough-devices-program
- Notice with Comment – Transitional Coverage for Emerging Technologies (CMS-3421-NC) | CMS. Accessed January 31, 2024. https://www.cms.gov/newsroom/fact-sheets/notice-comment-transitional-coverage-emerging-technologies-cms-3421-nc
- Farmer SA, Fleisher LA, Blum JD. The Transitional Coverage for Emerging Technologies Pathway—Enhancing Innovation While Establishing Patient Safeguards. JAMA Health Forum. 2023;4(8):e232780. doi:10.1001/jamahealthforum.2023.2780
- New prior authorization reforms show power of physician advocacy. American Medical Association. Published March 14, 2024. Accessed March 22, 2024. https://www.ama-assn.org/practice-management/prior-authorization/new-prior-authorization-reforms-show-power-physician
- CPT® code change applications. American Medical Association. Published April 15, 2024. Accessed April 17, 2024. https://www.ama-assn.org/practice-management/cpt/cpt-code-change-applications
- Artino AR, Youmans QR, Tuck MG. Getting the Most Out of Surveys: Optimizing Respondent Motivation. J Grad Med Educ. 2022;14(6):629-633. doi:10.4300/JGME-D-22-00722.1
Conflict of Interest
Dr. Ruggles reported personal fees from Summit Rock Strategy Consulting, Inc (consulting employment and ownership), minority equity from 3NT Medical, BioTrace Medical, Orthini, LLC, and employee stock grants from Acclarent/Johnson & Johnson outside the submitted work; and ongoing nonfinancial relationships with individuals trained at the Stanford Byers Center for Biodesign and others involved in advancing new medical technologies into patient care (e.g., venture investors, corporate leaders, industry associations, and service providers).
Dr. Makower reported personal fees over the past several years from New Enterprise Associates, Patient Square Capital, Elevage, Sofinnova Partners, minority equity and board membership with ExploraMed, Willow Innovations, Revelle Aesthetics, Moximed, X9, Allay Therapeutics, Setpoint Medical, minority equity and former board membership with Intrinsic Therapeutics, minority equity and board membership with Magenta Medical, minority equity in Moon Surgical, Cardionomic, Cala Health, CVRX, Ancora, Starlight Cardiovascular, Candescent Biomedical, iRhythm, former minority equity and former board membership with NeoTract/Teleflex, Acclarent/JNJ, Vesper Medical, Intact Medical, former minority equity from Ivantis and minority equity and former board member with Eargo and DOTS Devices outside the submitted work; in addition, Dr. Makower has more than 300 US patents issued in a wide array of fields related to the companies listed above—no additional financial consideration is associated with these patents. The authors are further supported by unrestricted donations to Stanford University, Stanford Byers Center for Biodesign and Stanford Biodesign Policy Program. Further, Dr. Makower had ongoing nonfinancial relationships with individuals trained at the Stanford Byers Center for Biodesign and others involved in advancing new medical technologies into patient care (e.g., venture investors, corporate leaders, industry associations, and service providers). No other disclosures were reported.